RESEARCH
CARBOHYDRATE
Carbohydrates are the richest sources of chiral feedstocks abundantly available from natural sources. The research programmes encompass,
- Monosaccharide modifications by identification of newer synthetic methodologies and reactions.
- Studies of intricate carbohydrate-protein interactions and the sources of the multivalency in these interactions.
- Studies of synthetic oligosaccharide glycolipids as cell surface mimics towards development of inhibitors of bacterial pathogens.
- Studies of cyclic oligosaccharides.
- Studies of materials properties, particularly, as liquid crystals. Click the hyperlink to view finer details of the advancements arising from these topics.
(i) Monosaccharide modifications: Hydroxy functionality-rich monosaccharides provide valuable source to implement varied types of chosen reactions. The reactions below give a glimpse of advancements made in monosaccharide modifications.
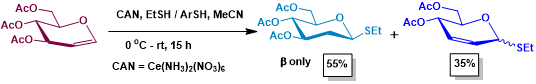
Figure 1. Addition vs Ferrier rearrangement in glycals, catalyzed by CAN.
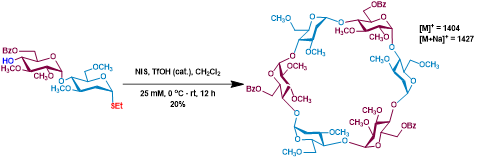
Figure 2. AB-Type disaccharide sugar monomer for cyclization to new cyclic 2-deoxy-oligosaccharides.
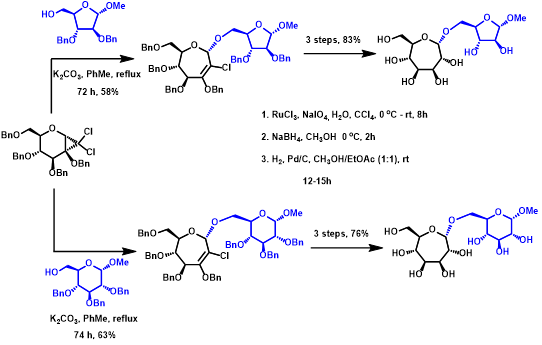
Figure 3. Ring expansions as rich opportunity to derive un-natural septanoside sugars.
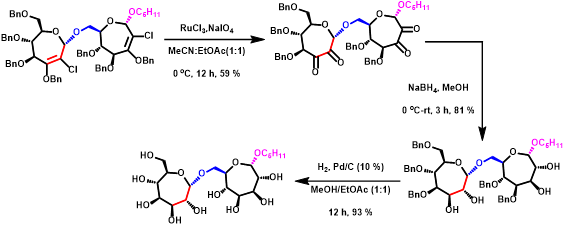
Figure 4. Setanosyl-septanoside disaccharide formation from un-saturated intermediate.
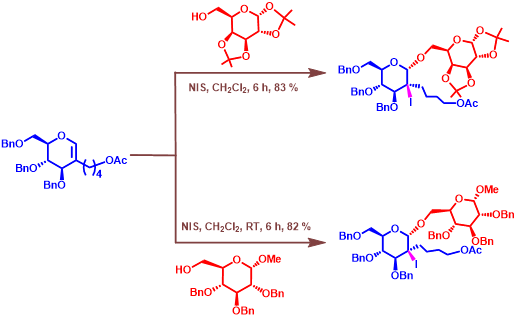
Figure 5. Glycosylations of 2-deoxy-2-C-glycal with sugar acceptors.
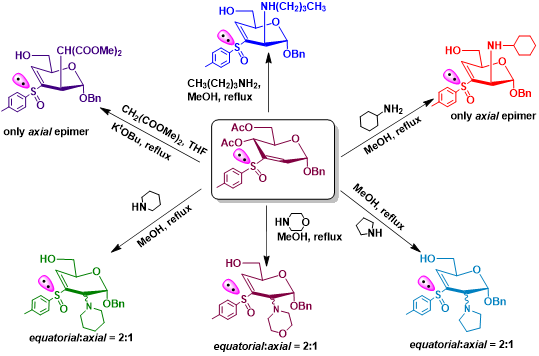
Figure 6. Reactions of 2,3-unsaturated sugar vinyl sulfoxides with varied nucleophiles.
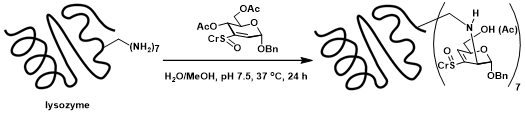
Figure 7. Glycoconjugation of sugar vinyl sulfoxide at the lysine sites of protein lysozyme.
(ii) Studies of intricate carbohydrate-protein interactions and the sources of the multivalency in these interactions: Uncovering the central role of the multivalency in carbohydrate-protein interactions is a main stay in Group’s research. Following figures give representative structures and methods used in the studies.
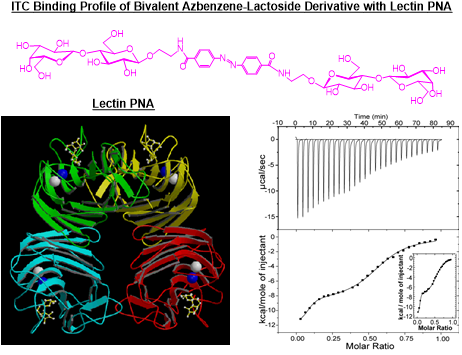
Figure 8. Bivalent lactoside binding to lectin PNA affords a biphasic binding profile.
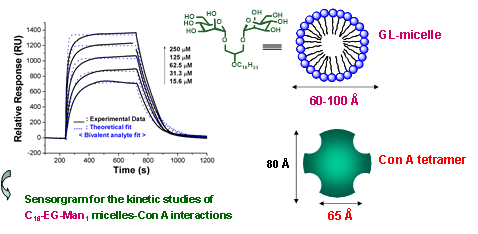
Figure 9. Kinetics of the bivalent glycolipid micelle with lectin Con A, as assessed by SPR technique.
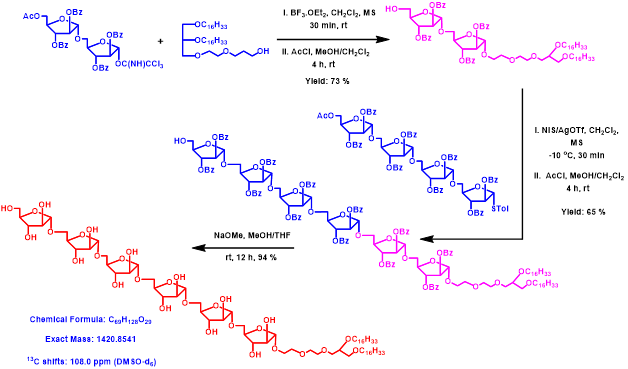
Figure 10. Synthetic hexasaccharide arabinofuranoside glycolipid for studies as inhibitor of mycobateria

Figure 11. Interaction of trisaccharides 3 and 4, with surfactant protein A, as assessed by SPR method.
(iii) Studies of synthetic oligosaccharide glycolipids as cell surface mimics towards development of inhibitors of bacterial pathogens: Mycobacterial cell surfaces are enriched with varied types of glycolipids, prominent among them are lipoarabinomannan and arabinogalactan. Sustained research are conducted to develop arabinomannan lipids, that present inhibition properties of the enzymes responsible for lipoarabinomannan biosynthesis. Following figures show the synthetic arabinomannan lipids and biochemical studies, conducted in collaboration with Prof. Dipankar Chatterji, MBU, IISc.

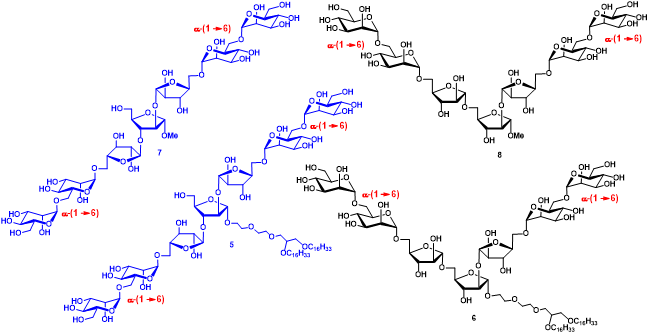
Figure 13. Synthetic arabinomannan heptasaccharides as cell surface glycolipid mimics.
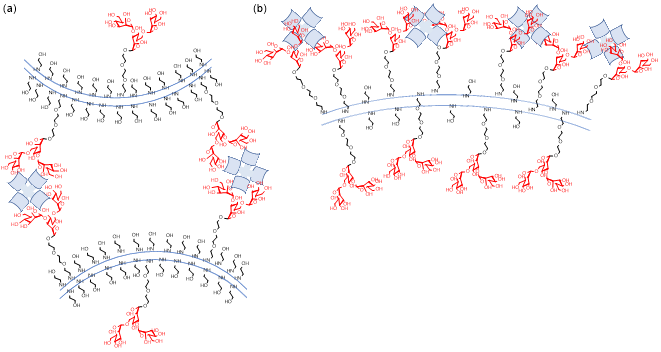
Figure 14. Synthetic glycolipid polymers possessing varying sugar ligand densities that differentiate cis- (intra-) and trans- (inter-) interactions with lectins.
(iv) Studies of cyclic oligosaccharides: Cyclic oligosaccharides are beneficial in a number of chemical, biological and materials studies. The class of cyclodextrins is available in plenty from enzymatic transformation of starch. Whereas chemical synthesis of cyclic oligosaccharides continues very few in literature at large. A focused research project of the group pertains to the synthesis of glycosidic bond-expanded cyclic oligosaccharides. The following figure provides an example of that of glycosidic-bond expanded cyclic pentasaccharide and solid state structure of a related cyclic trisaccharide.
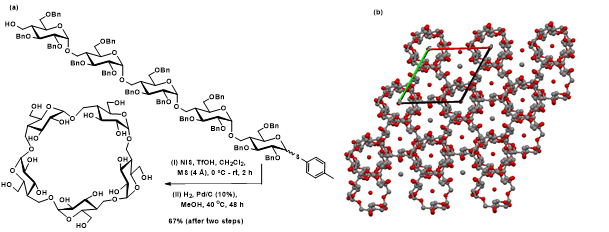
Figure 15. (a) Formation of glycosidic bond expanded cyclic pentasaccharide from the linear precursor. (b) Crystal structure packing diagram in the case of cyclic trisaccharide (right).
(v) Studies of carbohydrate liquid crystals: Amphiphilic, neutral carbohydrate liquid crystals possess interesting mesophase behavior, depending on the constitutions. The mesophase properties is relevant at the solid state and in solutions. A sustained research is advanced to identify the configurational relations of the amphiphilic glycolipids and the evolving mesophase properties. The following figure represents the anomeric constitution on the evolution of mesophase property or otherwise.

Figure 16. Double melting behavior of the b-anomer of a glycolipid, whereas the corresponding a-anomer forms thick network in the solid state.
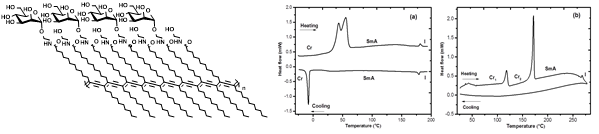
Figure 17. Polymeric sugar liquid crystals responding in a monotropic or enantiotropic phase changes, depending on the sugar constituent.
DENDRIMERS
Dendritic macromolecules are one of the very recent additions to the repertoire of synthetic macromolecules. Branches-upon-branches as the structural motif, dendritic macromolecules are by far the most monodispersed. Macromolecular nature coupled with complete branching throughout brings with it functional features not achievable easily with other types of macromolecules. Research on dendrimers encompass
- Development of monomers that lead to newer dendrimers, with varied types of reactive functionalities at their peripheries.
- Studies of surface functionalized dendrimers to identify the evolution of multivalent effects, particularly, in organometallic reactions.
- Evolving dendrimers as gene delivery agents, given the observations that the in-house built dendrimers are non-toxic and bio-compatible.
- Studies of clustered liquid crystalline mesogens on dendrimer scaffolds.
- Dendrimers as reagents for organic transformation. Click the hyperlink to view finer details of the advancements arising from these topics.
(i). PETIM and poly(alkyl aryl ether) dendrimers: These are two in-house built dendritic macromolecules. The figures below provide synthetic routes to prepare a generation of these dendrimers.
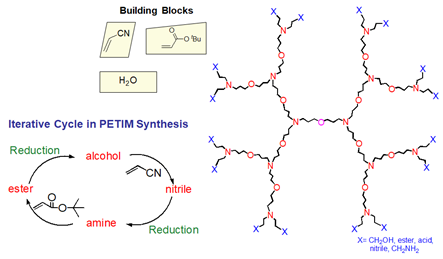
Figure 18. Iterative reactions leading to generation of poly(ether imine) (PETIM) dendrimers.
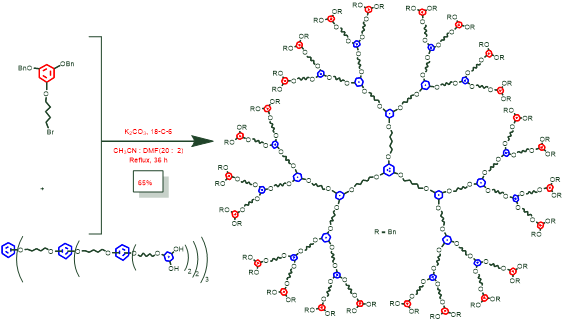
Figure 19. Synthesis of fourth generation poly(alkyl aryl ether) dendrimer.
(ii). Studies of multivalent effects, particularly in organometallic reactions: A possibility of knowledge transfer of multivalent effects seen commonly in biological systems to clustered organometallic catalysis is an active interest of the Group’s research activities. The following figures show typical multivalent organometallic catalysts, within one dendrimer generation, and results of the catalysis in chosen reactions.
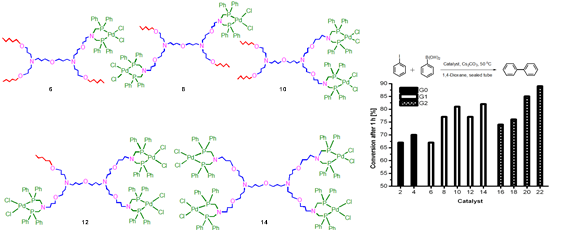
Figure 20. Dendritic organometallic complexes to identify multivalent effects in catalysis.
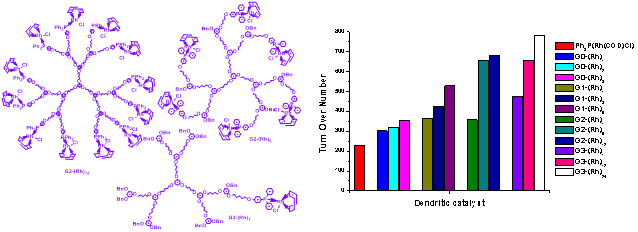
Figure 21. Dendritic Rh(I) catalysts in a hydrogenation reaction.
(iii). Dendrimers as gene delivery agents: The low or non-cytotoxic nature of PETIM dendrimers prompts their applicability in gene transfection studies. In collaboration with Prof. N. V. Madhusudans, NIMHANS, Bangalore and Prof. Saumitra Das, a series of biological studies shows that dendrimers condense DNA and RNA fragments that can be taken through gene transfection protocols to induce protein synthesis. In the given figure below, the green fluorescent protein synthesis is achieved using appropriate plasmid DNA complexed with the dendrimer.
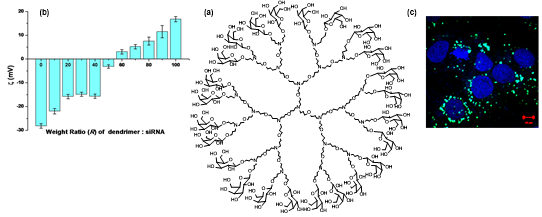
Figure 22. (a) PETIM dendrimer functionalized with galactose; (b) Zeta potential plot upon interaction with siRNA and (c) gene transfection on Huh7 liver cells.
(iv). Studies of clustered liquid crystalline mesogens on dendrimer scaffolds: Dendrimer scaffold provides functional effects on the mesogens that functionalize the peripheries of the dendrimers. An effect is observed difference in the smectic layering, where the lamellar layering is modulated, a modulated smectic A phase. The following figure represents molecular structures, observed microscopy picture and the model for the modulated smectic A phase. The studies are conducted in collaboration with Prof. S. Krishna Prasad and Dr. Shankar Rao, Centre for Nano and Soft Matter Science, Bangalore.
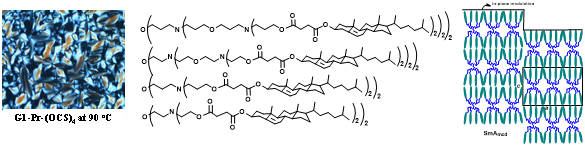
Figure 23. Cholesteryl-functionalized PETIM dendrimers showing a smectic A phase, within which the molecule organizes in a modulated lamellar organization.
(v). Dendrimers as reagents for organic transformations: Hydrolytic abilities change significantly when the hydrolysable moiety is present in different dendrimer generations. Taking advantage of this differing hydrolysis of moieties, organic reactions are conducted. The following figure shows that of an one-pot synthesis of tetra-aryl synthesis.
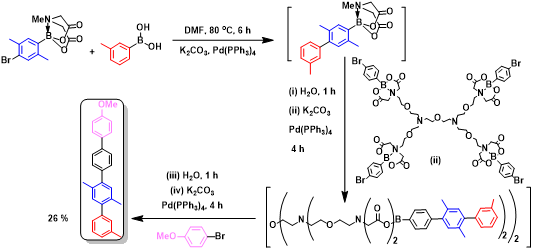
Figure 24. One-pot iterative SM coupling reaction, using dendritic catalysts.
Detailed studies of the above major topics is available in individual publications.
